Remdesivir

The move comes in light of the current epidemiological situation and the spike in coronavirus cases in India, the embassy said in a press release Saturday.
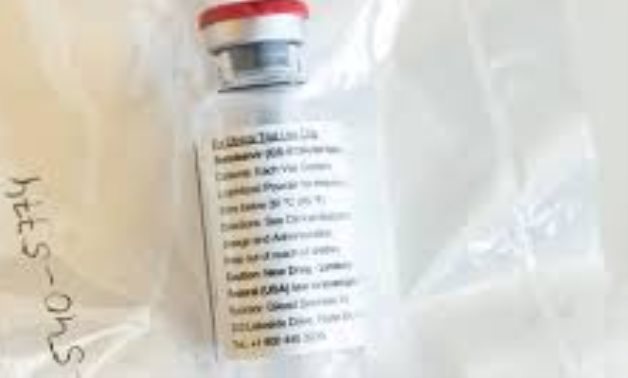
Gilead’s remdesivir should not be used for patients hospitalised with COVID-19.
Essam’s remarks come shortly before the European Commission announced on Friday granting conditional approval for antiviral Remdesivir for coronavirus treatment. Egypt has already started to produce the drug.
The number of coronavirus cases in Egypt during the past days has been stable, Minister of Higher Education Khaled Abdel Ghaffar, adding that this state will make the scenarios in the coming days more predictable.
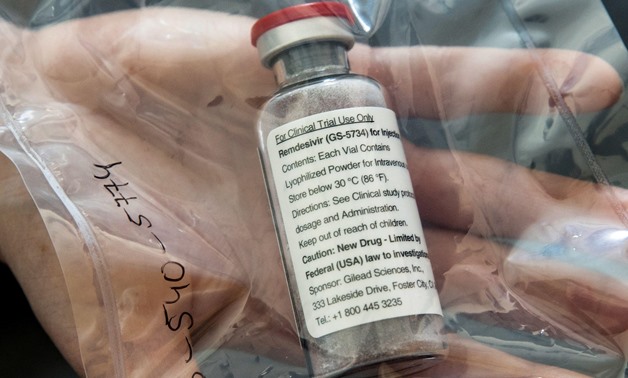
Egypt’s 23-year old drug manufacturer Eva Pharma announced starting producing antiviral <b>Remdesivir</b> as the first expected therapy for the novel coronavirus (COVID-19), Reuters reported.

Remdesivir was developed by the American biopharmaceutical company Gilead Sciences and has shown some good indications in coronavirus treatment.

The World Health Organization (WHO) confirmed that the first cargo of the medicine will be sent to Egypt.
Most Read